

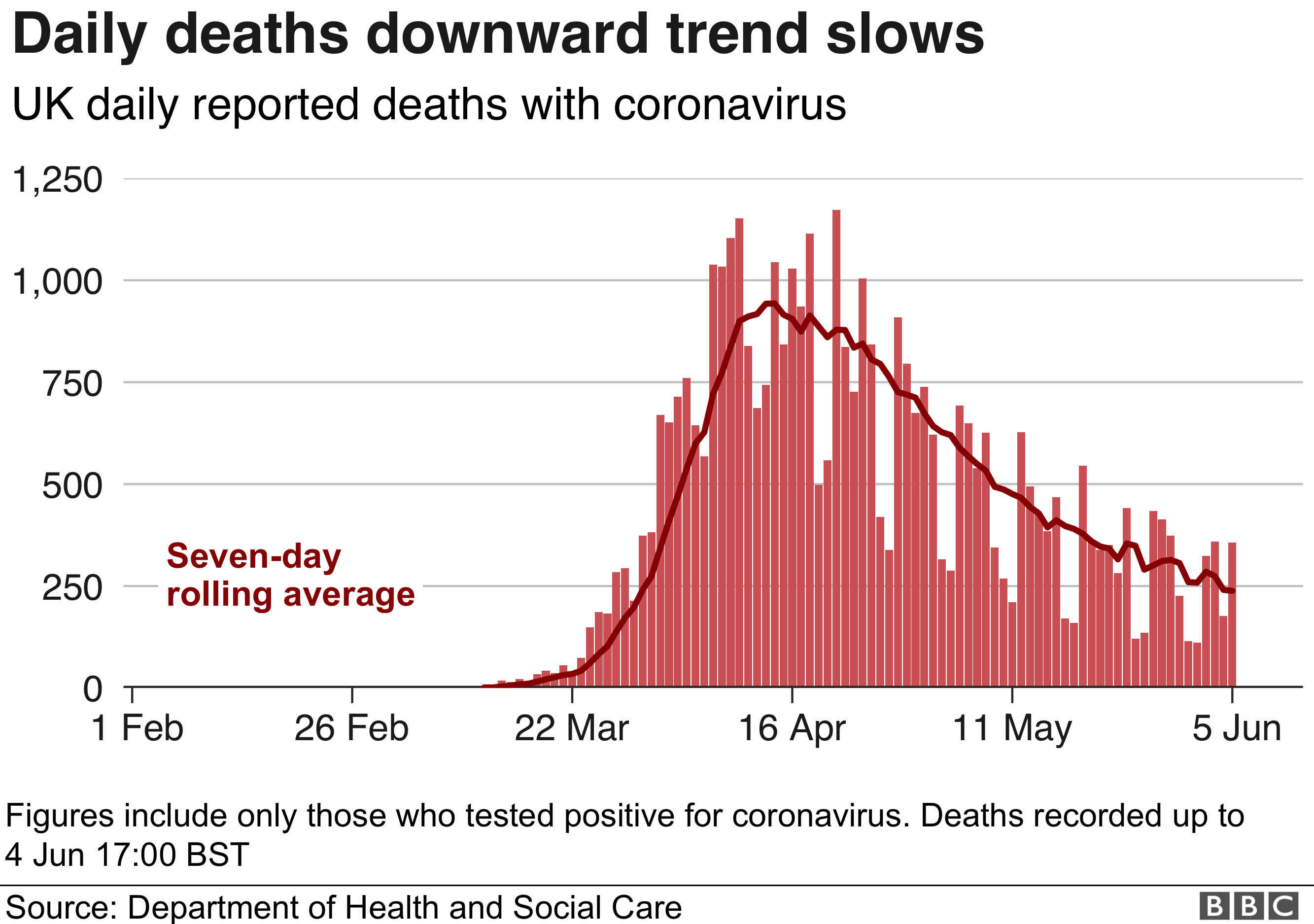
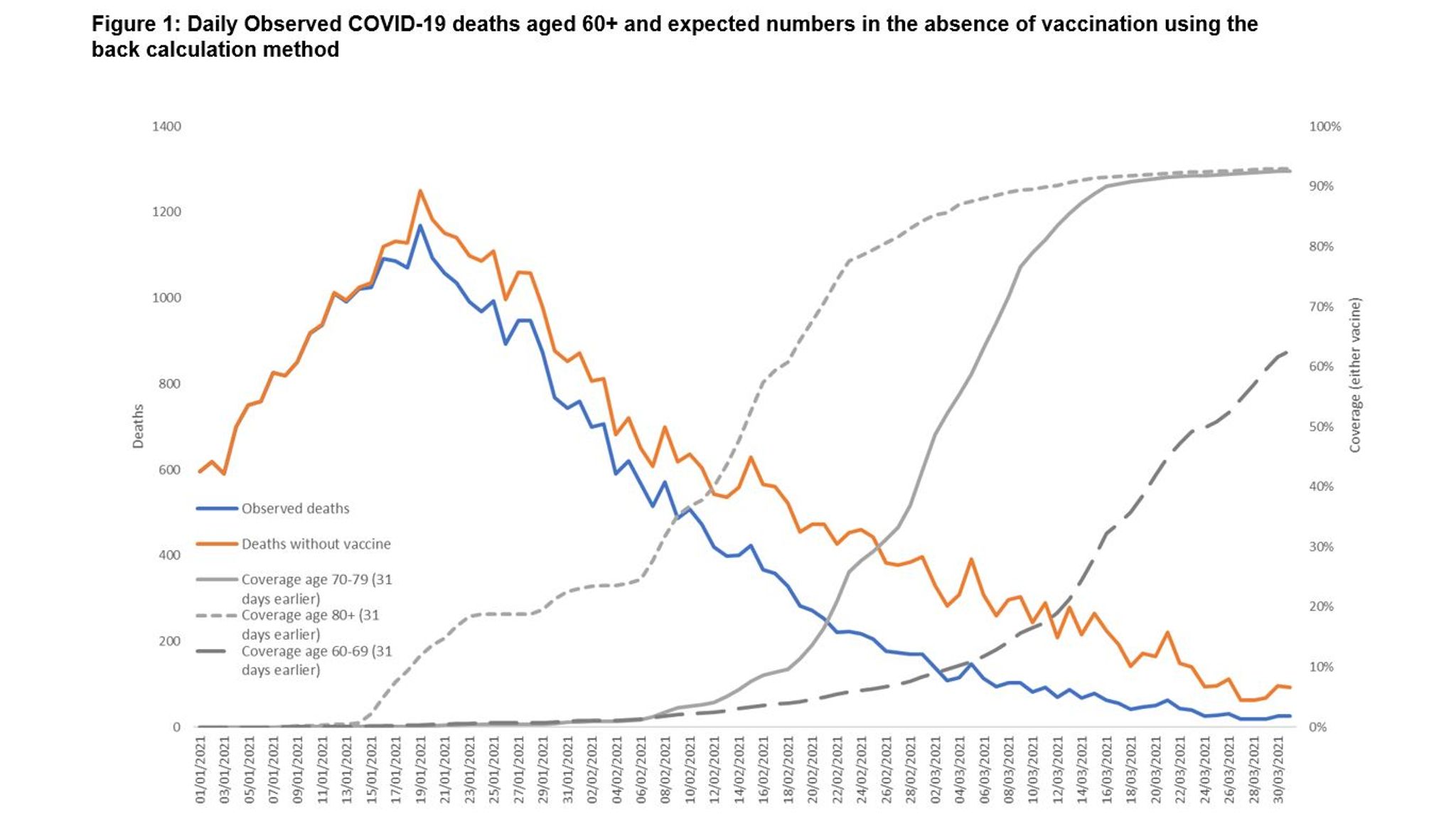
Information About COVID-19 EUAs for Medical Devices.Ventilators and Ventilator Accessories EUAs.Remote or Wearable Patient Monitoring Devices EUAs.Decontamination Systems for Personal Protective Equipment EUAs.Continuous Renal Replacement Therapy and Hemodialysis Devices EUAs.COVID-19 EUAs for Medical Devices, including:.COVID-19 EUAs for Drugs and Non-Vaccine Biological Products.Detailed Information for all COVID-19 EUAs, including authorizations and fact sheets.Coronavirus Disease (COVID-19) updates from FDA.Read more about what happens to EUAs when a public health emergency ends. Existing COVID-19 EUAs will remain in effect, and the agency may continue to issue new EUAs if the situation meets the criteria to do so. The ending of the COVID-19 PHE will not impact FDA's ability to authorize medical countermeasures for emergency use. In addition, in January 2014, FDA issued a question and answer document (PDF, 762K) to respond to questions raised by public health stakeholders about PAHPRA’s amendments to the EUA authority and establishment of new authorities related to the emergency use of MCMs during CBRN emergencies.Ĭoronavirus Disease 2019 (COVID-19) EUA InformationįDA expects the COVID-19 public health emergency (PHE) declared by the Department of Health and Human Services under the Public Health Service Act to expire on May 11, 2023. For more information, please see the JanuFederal Register notice. In January 2017, FDA finalized the guidance: Emergency Use Authorization of Medical Products and Related Authorities. From HHS: PREP Act Questions and Answers - COVID-19, including "How does the end of the PHE affect PREP Act coverage for COVID-19 countermeasures?".Fact Sheet: HHS Announces Intent to Amend the Declaration Under the PREP Act for Medical Countermeasures Against COVID-19 (April 14, 2023).Advisory Opinion 02-02 on the PREP Act and the Secretary's Declaration under the Act (PDF, 278 KB, May 19, 2020).COVID-19 PREP Act Declarations and Amendments (HHS).Notice of Declaration under the Public Readiness and Emergency Preparedness Act for medical countermeasures against COVID-19 (February 4, 2020).The Secretary issued this amendment to clarify that COVID-19 continues to pose a credible risk of a future public health emergency, add two new limitations on distribution, extend the time period of coverage for certain Covered Countermeasures and Covered Persons, clarify the time period of coverage for Covered Persons authorized under the Declaration, and extend the duration of the Declaration to December 31, 2024. On May 9, 2023, HHS Secretary Becerra signed the 11th amendment to the declaration under the PREP Act for COVID-19 Medical Countermeasures.
The HHS Secretary has issued several Declarations pursuant to section 319F-3 of the PHS Act to provide liability immunity for activities related to medical countermeasures against COVID-19. The PREP Act amended the Public Health Service Act (PHS Act) to add section 319F-3 (42 U.S.C. Information on the PREP Act can be found here. Public Readiness and Emergency Preparedness Act (PREP Act) Information on terminated and revoked EUAs can be found in archived information.
Subsequent HHS declarations supporting use of EUAs and based on this determination are described in the blue boxes below. On February 4, 2020, the HHS Secretary determined that there is a public health emergency that has a significant potential to affect national security or the health and security of United States citizens living abroad, and that involves the virus that causes COVID-19. Please note: a determination under section 319 of the Public Health Service Act that a public health emergency exists, such as the one issued on January 31, 2020, does not enable FDA to issue EUAs. The HHS declaration to support such use must be based on one of four types of determinations of threats or potential threats by the Secretary of HHS, Homeland Security, or Defense. Under section 564 of the Federal Food, Drug, and Cosmetic Act (FD&C Act), when the Secretary of HHS declares that an emergency use authorization is appropriate, FDA may authorize unapproved medical products or unapproved uses of approved medical products to be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions caused by CBRN threat agents when certain criteria are met, including there are no adequate, approved, and available alternatives.


 0 kommentar(er)
0 kommentar(er)
